Overview and Objectives
BLISS project aims to tackle antimicrobial resistance by creating novel BLIs to restore the antibiotic activity of commercially available β-lactams. BLISS focuses on three main objectives and aims to lead to the development of one or more potential drug candidates.
Objective 1. Investigate the use of KPC-2 as scaffold for in situ click chemistry and evaluate its ability to generate triazole-based BATSI.
Objective 2. Discover novel inhibitors for KPC-2 using KTGS.
Objective 3. Identify at least one highly active BATSI against clinically relevant β-lactamases to be tested in vivo.
Inside BLISS
Antimicrobial resistance, β-lactamases and β-lactamase inhibitors
Antimicrobial Resistance (AMR) refers to the inability of bacteria, viruses, fungi and parasites to respond to conventional treatment and drugs. As a result of AMR, infections caused by drug resistant microbes often results in severe illness and death for hospitalised patients. Emergence and worldwide spreading of drug-resistant pathogens expressing new resistance mechanisms, combined to the drought on the pipeline for new antimicrobials, makes AMR a social and economic threat.
Microbes evolve constantly, mainly through genetic mutations. For example, since the introduction of penicillin in the market, Gram negative (Gram-) bacteria developed resistance towards β-lactam antibiotics. β-lactam are the most popular antibiotic class and act by inhibiting the biosynthesis of the bacterial cell wall. The main mechanism used by bacteria to avoid the pharmacological activity of β-lactams consists in the expression of β-lactamases, a class of enzymes (EC 3.5.2.6) that are either chromosomally encoded in a species or plasmid-mediated.
β-lactamases hydrolyse β-lactam rings, therefore inactivating the antibiotic and causing resistance. β-lactamase are characterized by a wide structural variety with over 7500 types reported by the β-lactamase database (http://www.bldb.eu/). The Ambler classification categorise β-lactamase into four classes: the active-site serine β-lactamases (SBLs, classes A, C and D) and the zinc-dependent or metallo-β-lactamases (MBLs; class B). SBLs hydrolyse β-lactams through a catalytic serine located in their active site in a 2 steps acylation/diacylation mechanism, whereas MBLs employ Zn2+ ions to coordinate water for the hydrolysis process.
Among the different types of SBLs and MBLs, the extended spectrum β-lactamases (ESBLs) and carbapenemases are the most worrying subclasses and are posing a major therapeutic challenge in the treatment of hospitalized and community-based patients. ESBLs are plasmid-mediated enzymes that hydrolyses penicillins, cephalosporins (first- to third-generation) and aztreonam. They are inhibited by β-lactamase inhibitors (BLIs), including clavulanic acid. On the other hand, carbapenemases are the most resilient subclass of β-lactamases possessing the widest spectrum of activity, incorporating also carbapenems as their target, which are often defined the “last resort” antimicrobials in hospitals and long-term care facilities.
The most effective carbapenemases families included:
- – KPC family (Klebsiella Pneumoniae Cephalosporinase, class A)
- – OXA family (Oxacillinases, Class D)
- – VIM family (Verona Integron-encoded Metallo-β-lactamase, Class B)
- – IMP family (Imipenemase, Class B)
- – NDM family (New Delhi Metallo-β-lactamase, class B)
To fight the spread of β-lactamases and restore the pharmacological activity of β-lactams, β-lactamase inhibitors (BLIs) have been developed. BLIs can be divided in three main categories based on their core structure:
- – BLIs containing a β-lactam core (i.e. Clavulanic acid, Sulbactam, Tazobactam)
- – BLIs containing a β-lactam possessing a diazabicyclooctane core (i.e. Avibactam and Relebactam)
- – BLIs with a boronic acid core (i.e. Vaborbactam)
Our group main work focuses on the discovery of novel BLIs with a boronic acid core, defined as Boronic Acid Transition State Inhibitor (BATSI).
Related links and publications:
1) https://www.who.int/health-topics/antimicrobial-resistance
3) https://health.ec.europa.eu/antimicrobial-resistance/eu-action-antimicrobial-resistance_en
4) Tooke, C. J. et al. J. Mol. Biol. 2019. 431, 3472–3500. DOI: 10.1016/j.jmb.2019.04.002
5) Hammoudi Halat, D. et al. Antibiotics 2020. 9, 186. DOI: 10.3390/antibiotics9040186
6) González-Bello, C. et al. J. Med. Chem. 2020. 63, 1859-1881. DOI: 10.1021/acs.jmedchem.9b01279
Developing novel boronic acid transition state inhibitors (BATSI) as β-lactamase inhibitors
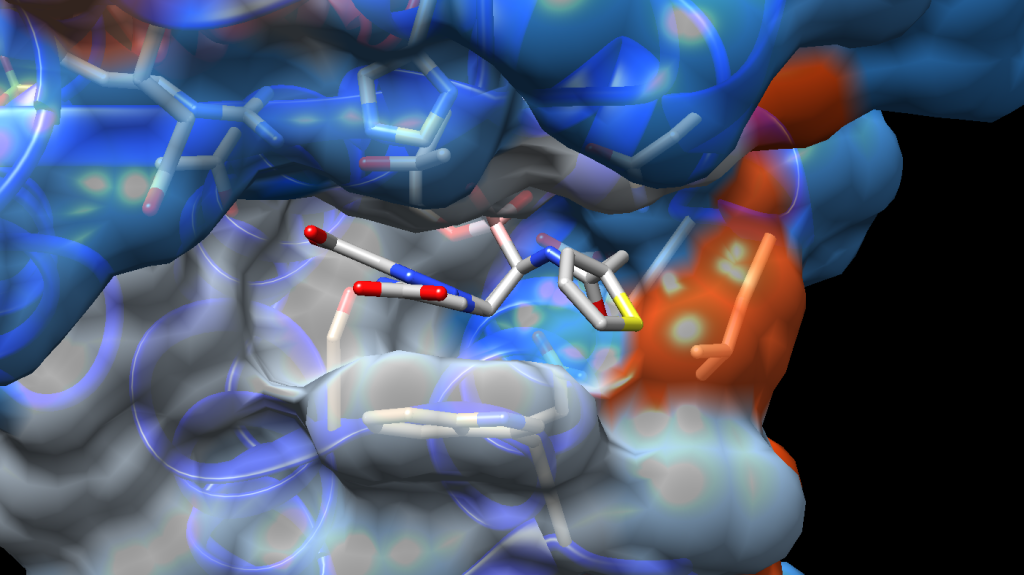
BATSIs act through the formation of a reversible covalent bond between the boron atom and the catalytic serine residue of SBLs leading to the enzyme inhibition and β-lactam activity restoration. Similarly, MBLs can be inhibited by the boronic acid core of BATSI through coordination of one or two Zn2+ ion(s) located in the active site. Being a novel chemical identity for the bacteria, boronic acid transition state inhibitors (BATSIs) induce pre-existing mechanism of resistance and form a stable enzyme-inhibitor complex that inactivate the β-lactamase target.
Vaborbactam, a cyclic boronic acid specifically designed for the inhibition of β-lactamases, has been the first and only boronic acid transition state inhibitor (BATSI) to be approved by regulatory authorities. Commercialised under the name VABOMERE®, Vaborbactam is employed for treatment of bacterial infections in co-administration with the β-lactam antibiotic meropenem.
Since then, the research of novel β-lactamase inhibitors (BLIs) containing the boronic acid motif has produced only two other clinical candidates, Taniborbactam (VNRX-5133) and Xeruborbactam (QPX-7728). In the past twenty years, Caselli’s and Prati’s groups dedicated their work to discover new BLIs, focusing on the creation of boronic acids with inhibitory activity towards clinically relevant β-lactamases. Their seminal work, covered by several publications in prestigious peer-reviewed journals, led to the development of a series of compounds exhibiting potent biological activity for serine β-lactamases.
Starting from the design of inhibitors inspired by the most common β-lactams and moving progressively towards a biomimetic approach, Prati/Caselli groups developed several BATSIs with high activity and selectivity towards a broad spectrum of serine β-lactamases.
Relevant Work by Caselli’s and Prati’s groups:
- Eidam O. et al. J. Med. Chem., 2010. 53, 7852–7863. DOI: 10.1073/pnas.1208337109
- Caselli E. et al. J. Med. Chem., 2015. 58, 5445−5458. DOI: 10.1021/acs.jmedchem.5b00341
- Nguyen N. Q, et al. Antimicrob. Agents Chemother. 2016. 60, 1760–1766. DOI: 10.1128/AAC.02643-15.
Kinetic Target-Guided Synthesis (KTGS) role as platform for drug discovery
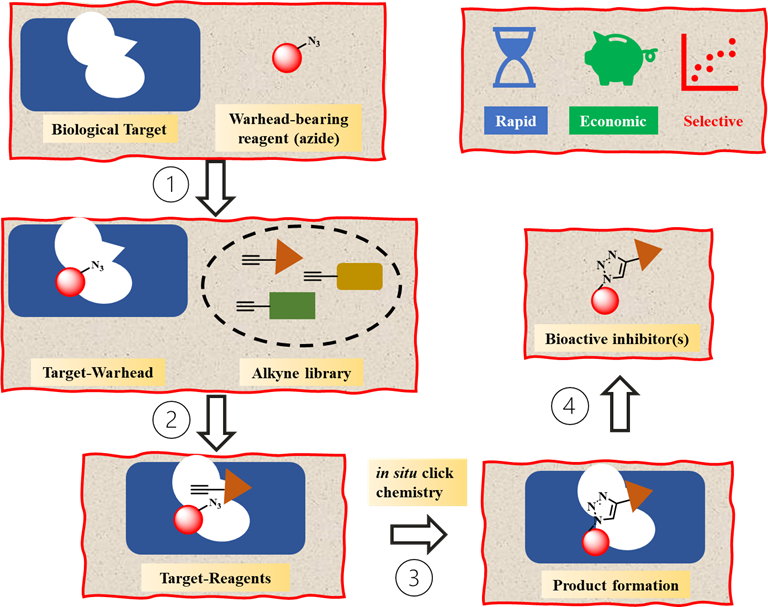
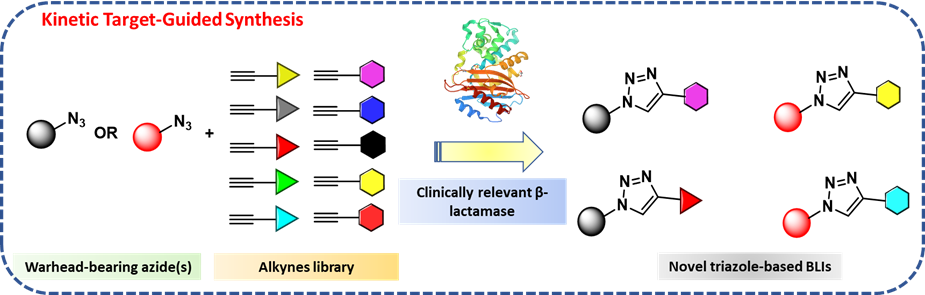
Kinetic Target-Guided Synthesis (KTGS) is a unique strategy for drug discovery where a biological macromolecule is employed to catalyse the synthesis of its own inhibitors from a library of reagents. In KTGS, “warhead-bearing reagents” are often used to drive the reaction within the biological target, i.e. enzymes, therefore allowing the target active site to discriminate among a selection of ligands with the best electronic and/or steric properties.
KTGS offers several advantages as approach for drug discovery. Whether KTGS is used for hit-to-lead optimisation or to find active inhibitors for proteins with unknown conformation, it often allows a quick and efficient screening of several small-molecules. Moreover, most ligands used in KTGS are conform with “possible to be oral chemical space” properties. Hence, KTGS has peculiar characteristics that facilitate the investigation of a broader chemical space than the Ro5 space and expedite the process for hit optimization. To this extent, the inner biocompatibility of alkynes and azides, combined to their easy synthetic access and commercial availability, has favoured the development of in situ click chemistry.
The versatility and selectivity of in situ click chemistry has allowed the identification of several hit compounds for various biological targets. The tremendous potential of KTGS, and more specifically of in situ click chemistry, has not been exploited for the development of BLIs yet. To fully exploit the potential of boronic acids as antibacterial agents and expedite the drug development process, BLISS pioneers KTGS as a powerful tool for the development of BLIs.
Relevant Work on KTGS and in situ click chemistry:
- Bosc, D. et al. J. Med. Chem., 2020. 63, 3817–3833. DOI: 10.1021/acs.jmedchem.9b01183
- Camberlein, V. et al. Angew. Chem. Int. Ed., 2022. 61, e202203560. DOI: 10.1002/anie.202203560
- Bhardwaj, A. et al. Nat. Commun. 2017. 8, 1. DOI: 10.1038/s41467-016-0009-6
